Abstract
Epigenetic regulators function in a context-dependent manner and are commonly mutated in tumors. Activating mutations and overexpression of the histone-methyltransferase, enhancer of zeste, homolog 2 (EZH2), occur in lymphoma and other malignancies, whilst loss-of-function mutations are predicted in myeloid malignancies. We study this apparent contradiction, examining the importance of cellular context and disease stage for Ezh2 loss, genetically deleting Ezh2 during the induction or maintenance of a single malignancy, Acute Myeloid Leukemia (AML).
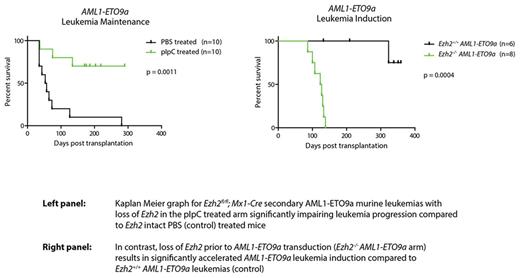
We initially focused on the role of Ezh2 in AML maintenance, using conditional Ezh2fl/fl mice. Following in vitro retroviral immortalization of haematopoietic stem and progenitor cells (HSPCs) from these with AML-fusion oncogenes: MLL-AF9 or AML1-ETO9a (reflecting two separate models of AML clinically associated with good and poor prognosis, markedly differing in mechanism of leukemic transformation and representing AML heterogeneity), these were then transduced with p-babe-Cre-puro vector (or empty vector) to delete Ezh2 . Strikingly, Ezh2 loss completely abolished cellular growth and colony formation, whilst empty vector transduced cells maintained their clonogenic output. To interrogate Ezh2 as a target in AML maintenance in vivo, we generated secondary MLL-AF9 or AML1-ETO9a leukemias through transplantation of bulk leukemia derived from Ezh2fl/fl ; Mx1-Cre primary leukemic mice. Following engraftment, deletion of Ezh2 was achieved by pIpC administration. Loss of Ezh2 in vivo markedly impaired both secondary MLL-AF9 and AML1-ETO9a leukemia progression. These data suggest that Ezh2 is an oncogene across multiple disparate subtypes of established AML and propose it as a possible therapeutic target in this context .
Addressing this hypothesis, we examined the role of pharmacological inhibition of EZH2 in AML, utilizing GSK343, an S-Adenosyl Methionine competitive EZH2 inhibitor. Through colony formation and functional assays, we demonstrated in vitro sensitivities of MLL-AF9 transformed murine BM HSPCs, primary murine Ezh2+/+ MLL-AF9 tumours , the AML1-ETO fusion driven human cell line Kasumi-1 and primary leukemic cells isolated from patients with various AML genotypes to GSK343 at similar IC50 (~10µM) across all cells. In contrast, minimal effects of GSK343 were seen on normal primary CD34+ HSCs clonogenic function. Together these data provide evidence of therapeutic potential, validating EZH2 as a target in AML.
To characterise the role of Ezh2 during AML induction in vivo, we utilized Ezh2fl/fl; Mx1-Cre mice to generate Ezh2-/- mice and compared them to Ezh2+/+controls. HSPCs from these were retrovirally transduced with MLL-AF9 or AML1-ETO9a and transplanted into lethally irradiated wild-type recipients, and leukemias allowed to develop. Remarkably in the context of disease induction, and in stark contrast to the maintenance experiments, Ezh2 functioned as a tumor suppressor gene, with its loss significantly accelerating leukemia generation across both subtypes of AML (compared to control).
To interrogate the molecular mechanisms of these findings we performed integrated genomic analysis with differential global gene expression analysis via RNA-sequencing (in Ezh2+/+ vs Ezh2-/- non-transformed HSPCs, Ezh2+/+ vs Ezh2-/- AML1-ETO9a and Ezh2+/+ vs Ezh2-/- MLL-AF9 leukemias) and ChIP-sequencing of the histone modifications H3K27me3 and H3K4me3. These revealed that during disease induction, Ezh2 represses a subset of bivalent promoters that resolve towards gene activation upon Ezh2 loss, inducing a number of genes including the transcription factor Plag1 (with known oncogenic function in CBFB-MYH11 murine leukemogenesis). Of note, overexpression of Plag1 phenocopied Ezh2 loss i n vivo to significantly accelerate MLL-AF9 leukemia induction.
In addition, we demonstrated virtual mutual exclusivity of genes de-repressed in established MLL-AF9 leukemias following pharmacological Ezh2 inhibition compared to those de-repressed in the induction of Ezh2-/- MLL-AF9 leukemias. This de-repression of entirely separate gene programmes explains the starkly contrasting phenotypic outcomes of targeting Ezh2 in AML induction and maintenance, and further provides reassurance that EZH2 inhibition in established AML is a safe strategy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.